Introduction to Photoelectric Effect - Photon Picture revisited
Atoms are made of negatively charged electron clouds bound to positively charged nucleus. In gases atoms move about in space almost independent of one another. Occasionally these lone atoms collide with one another and possibly exchange energies. The atoms in liquids behave similar to those in the gas phase, but now they regularly interact with one another. Still, as in the gas, atoms in fluids move about fairly freely. In solids atoms come relatively close to each other, but now they cannot move about. These atoms tend to balance the repulsive forces of the electron clouds of neighboring atoms with the attractive forces of electrons and atomic nuclei. In some conducting solids, such as metals, certain atomic electrons called valance electrons become free to move from one atomic site to another. But even these orphan electrons are bound to the solid as a whole, unless some external force free them and make them leave the metal. When light falls on a metal it can remove valance electrons from its surface and in this way create an electric current. This phenomena is called photoelectric effect.
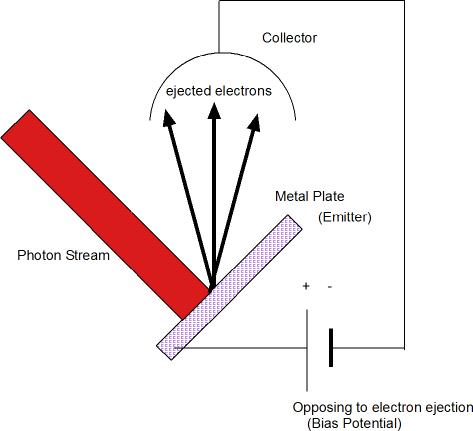
The above diagram shows the schematics of a typical photoelectric measuring apparatus. The metal emitter and metal collector are connected to a battery or power supply that is reverse biased. That it to say, its polarity is such that it inhibits ejection of electrons. As this applied electric potential varied, start of a measured electric current would signify the threshold voltage. This threshold voltage then determines the minimum energy required to free an electron from the metal emitter. In this way measurements can establish the binding energy of electrons on the metal surface.
Photoelectric effect was first discovered by Hertz in early 19th century, but its full explanation did not take place until Einstein applied Planck's photon picture of light to this problem. Experimentally observed aspects of this phenomena were:
- when light falls on the metal photo-electrons are emitted almost instantaneously, independent of how weak the light source is
- the photocurrent is directly proportional to the intensity of the light
- a bias retarding voltage reduces photocurrent to a zero value (this value of bias voltage is called the stopping voltage), for fixed light source
- the stopping voltage depends only on the frequency of the light and not on its intensity
If the only effect of the light was to provide extra energy for the electron to get eject from the metal, then one would expect that photocurrent would depend on the intensity of the light. The more light, the more energy it has to impart to the electron. But this was not at all what the experiment verified. Also, if the light was not intense enough, one would expect that it was a matter of time for the energy it provided that the electron would gain sufficient energy to leave the metal surface. But this also was not the case, as the process was almost instantaneous (occurred in 10 -9 s).
Einstein's explanation was based on Planck's radiation theory with a slight twist: light is quantized. Light of frequency f is made of quantized photons each of energy Ephoton = h fphoton , where h has a value of 6.63x10-34 J.s = 4.136x10-15 eV.s independent of the light, and is therefore known as the Planck's constant. So red light is made of photons that are weaker than the photons that make up blue light. A stream of red light, albeit of high intensity, may not have enough per photon energy to get an electron free from the metal surface. This is indeed what quantization is all about!
Physics of Color
In the photoelectric effect laboratory experiment one often uses a mercury lamp as a source of generating photons of different colors. When the light emitted by this lamp is put though a diffraction grating it is broken into individual and well separated color bands. Each color band is the same shape as the slit on which the light of the mercury lamp is imaged. In fact the shape of the band is the image of the illuminated slit. The colors differed in intensity. Some of the color bands (the red and the blue-green) are broad and diffuse whereas others (like the violet) are sharp and "well focused" image of the slit. These colors and their associated wavelengths are listed below:
color wavelength intensity
red 620.0 nm very weak and broad
yellow 578.0 nm clearly visible
green 546.1 nm brightest
blue-green 491.6 nm weak
blue 435.0 nm visible as violet
violet 404.7 nm violet and visible
UV 365.0 nm not visible
UV II 334.1 nm not visible
UV III 312.5 nm not visible
In the photoelectric experiment we investigate the effect of both the intensity and wavelength of the light on the production of electric current from a metal surface. We do not worry about what happened to the light that is not absorbed by the metal for the release of the photo-electrons. The questions that we are now going to ask have to do with the interaction of light with matter. What happens when light falls on material. In particular, what happens to its wavelength.
In the microscopic (atomic) picture all matter is made of atoms. Atoms can bind together to form molecules, as in the case of gas, or bind in groups to make solids. Metals, for example, are composed of atoms arranging themselves in a crystalline bound structure. Single atoms, due to their quantized energy structure, can absorb or emit photons of very distinct wavelengths. (Remember that photon energy is equal to Planck's constant times the frequency of the photon.) Each species of atom has its own set of quantized energy levels. As the atom changes its energy level it absorbs or emits the energy difference in the form of a photon. It is because of this quantization that the "spectrum" of our mercury lamp produced the above mentioned distinct set of colors. (It did not produce any orange line, for example, because mercury atom's energy level structure does not allow for such energy photon being absorbed or emitted.) The same type of quantization is also the case with molecules. However, the more complex the molecule, the more numerous its energy levels become. As a result, there are often numerous photon wavelengths that the molecule could absorb or emit. This process gets even more complex in the case of crystalline solids, whose energy levels get mangled to, instead, form energy bands. Solids could then emit or absorb photons of a continuum of wavelengths. In a way, for solids, quantization is not a major factor.
In the macroscopic picture when light falls on a substance it gets absorbed, transmitted, or reflected. How do these processes effect the intensity and wavelength of the light? Perhaps a better way of thinking of this macroscopic picture is to view all of these processes as one that always includes absorption followed by re-emission. The re-emission could involve photons of the same wavelength as those that were absorbed. Or, the re-emitted photon may have a different wavelength. Also, the re-emission could be in the original direction of propagation or in a random direction. In this way we could model mirror reflection of metal surfaces by stating that the light is absorbed by the metal and re-emitted with the same exact wavelength, but in a direction that satisfies the law of reflection. Likewise, a color filter could be modeled as a material that absorbs all wavelengths. It then re-emits all but one of the wavelengths as "reflection" and transmits the one wavelength that it does not reflect. There is a third possibility, however; namely the material can totally absorb the photon and not emit any! This happens when the energy of the absorbed photon is used to move the atoms about in the material (i.e. used to increase the kinetic energy of the atoms - heat up the material).
As it turns out, all of these processes do in fact contribute to account for the color of things. For example, in the case of the sky's color it is the absorption of the blue and UV wavelengths of the sun by molecules in air and their re-emission in all directions that makes it look blue. Red part of the sun's spectrum is transmitted by air and does not contribute to the color of the sky. (As we saw earlier, sun light is made of continuum of colors.) But in most cases the process that gives the color of objects is what is called: "selective absorption". In this process the material absorbs one or more wavelength of light. The reflected light will then be missing the absorbed color. Since white light is made of its component colors, when one of these components is selected out the remaining ones will no longer appear white. Water, for instance, has the color of greenish-blue because it absorbs red and IR wavelengths. Most organic dyes also absorb colors in the visible and through selective absorption appear their color.
A rather interesting case is that of white dyes. As it turns out, white dyes are not selective absorbers. To the contrary, white dyes do not absorb any of the colors. In paints these dyes are in fact small transparent grains or fibers that just reflect and refract light. Through multiple reflections from many small grains the light appears white because all wavelength undergo refraction and reflection - none is absorbed. This is why water vapor that is made of small transparent water droplets appear white. In white paints transparent inorganic oxides (titanium, zinc, or lead) are suspended in a fluid that has an index of refraction very different from the suspended grains. It is this difference in the index of refraction that enhances reflections at the grain boundaries to enhance the whiteness. If we wet a white cloth we decrease this difference in index and thus make the cloth look gray and less white.
Other processes that account for color of objects are diffraction and interference. We have already noticed how white light is turned into a rainbow of colors by reflecting from a diffraction grating. Soap films floating on water or in soap bubbles also make color rainbows by causing interference among reflected light of different wavelengths. Interestingly enough, animals also use interference property of light to color themselves! Examples of these are many: reptiles, insects, and birds. More on diffraction later!
Try the color mixing applete!
Last Modified: Wednesday, 12-Nov-2003 malekis@union.edu